|
| |
| -
2006 - |
| Jan. 12, 2006 |
Host: James Farrell,
University of Arizona
Presentation by: James Farrell, University of Arizona
Topic:
"Ab Initio
and Force Field Modeling of Physical and Chemical Adsorption Phenomena"
Abstract: Quantum
mechanical and force field based modeling is becoming increasingly utilized
for understanding adsorption mechanisms at the molecular scale. This talk
will discuss applications of force field based Monte Carlo, molecular
dynamics, and molecular mechanics modeling, as well as ab initio
quantum mechanical modeling, for studying the physical and chemical
adsorption of organic and inorganic compounds to reactive and nonreactive
surfaces. An introduction to each technique, the scope of problems that can
be addressed by each method, and the difficulties, limitations and
accuracies associated with each type of modeling will be discussed. Specific
examples will look at adsorption and transport of trichloroethylene (TCE) in
silica micropores, chemical adsorption and reaction of TCE with iron
surfaces, and chemical adsorption of arsenite species on ferric hydroxides.
(PDF) |
| Jan. 26 |
Host: Srini Raghavan, Materials
Science and Engineering, University of Arizona
Presentation by:
Topic: "Anodic Dissolution Of Copper
In Dilute Hydroxylamine Solutions - Applications To ECMP Of Copper"
Abstract: The effect of anodic polarization on
dissolution of copper in hydroxylamine based chemistries has been studied to
evaluate the use of these chemistries in an ECMP process. The dissolution
rate of copper in hydroxylamine solutions is pH dependant and exhibits a
maximum value with respect to over potential. In this study, the
effectiveness of benzotriazole (BTA) in inhibiting copper dissolution in
hydroxylamine based solution was tested using a Quartz Crystal Microbalance
(QCM) technique. Copper was electroplated onto the gold electrode of quartz
crystals and its dissolution/passivation behavior in hydroxylamine system
was studied at different applied potential values. Using electrochemical
polarization measurements in conjunction with QCM tests, the mechanism of
copper passivation has been studied. (PDF) |
| Feb. 9 |
Host: Jim Field, University of
Arizona
Presentation by: Jim Field, University of Arizona
Topic: "Biodegradation of Halogenated Solvents"
Abstract: Chlorinated solvents such as chlorinated ethenes, ethanes and methanes are important priority pollutants of
groundwater. The objective of this presentation is to summarize a
comprehensive literature review on the microbial degradation of chlorinated
solvents (Field and Sierra, 2004). A few examples will also be reviewed with
fluorinated solvents. Diverse strategies are utilized by microorganisms in
the degradation of organohalogen compounds ranging from reductive
dehalogenation, hydrolytic to oxygenolytic release of halides. To understand
how microorganisms gain energy and benefit from biodegradation of
halogenated solvents, one must consider biodegradation as a redox reaction
in which an electron donor becomes oxidized at the expense of an electron
acceptor becoming reduced as follows:
Electron Donor + Electron Acceptor ®
Oxidized Electron Donor + Reduced Electron Acceptor.
Lower halogenated compounds typically serve as an electron donor and carbon
source by being oxidized if an adequate electron acceptor is present (e.g.
O2). On the other hand, higher halogenated compounds can serve as
electron acceptors by being reductively dehalogenated in the presence of
electron donating compounds (e.g. H2 or simple organic
substrates) in a process known as halorespiration. Halogenated solvents can
also be cometabolically biotransformed by accidental reactions with enzymes
and cofactors involved in the degradation of other substrates. Classic
examples of cometabolism include oxidation of chlorinated solvents by
monooxygenases and reduction of chlorinated solvents by common-occurring
reduced cofactors in anaerobes (vitamin B12). The essence of bioremediation
is to provide environmental conditions that favor the reaction required such
as the supply of electron donor, electron acceptor or cosubstrate.
The bioremediation of perchloroethylene (PCE) and trichloroethene (TCE)
involves halorespiration. There is a high level of biodiversity for the
initial reductive reactions to cis dichloroethene (cDCE) thus these
reactions are ubiquitous as long as electron donors are supplied or
otherwise present as co-contamination at the site. However, the final
reductive steps to vinyl chloride (VC) and ultimately ethene are restricted
to halorespiring bacteria from the genus Dehaloccocoides which are
not universally present at all sites. If absent, the bioremediation can
potentially benefit from inoculating Dehaloccocoides. An alternative
approach to PCE bioremediation, is to provide a sequence of anaerobic and
aerobic degradation steps. First reduction of PCE to cDCE is stimulated by
addition of electron donors, thereafter the cooxidation of cDCE can be
promoted by providing oxygen and substrates that enrich for organisms with
monooxygenases (e.g. methane, phenol etc.). Aside from
cooxidation, several strains of aerobic bacteria have recently been
discovered that can utilize cDCE and VC as growth supporting primary
substrates.
The bioremediation of carbon tetrachloride (CT) involves cometabolic
reductive dechlorination reactions. A large number of cofactors have been
shown to be involved in CT biotransformation ranging from vitamin B12 to
quinones. The main reaction involves CT reduction to a dichlorocarbene
radical, which is hydrolyzed by water to form CO and/or formate that are
subsequently degraded further to CO2 in the absence of oxygen.
[Field, J.A. & R. Sierra-Alvarez (2004) Biodegradability of chlorinated
solvents and related chlorinated aliphatic compounds. Reviews in
Environmental Science & Bio/Technology 3:185-254.]
(PDF) |
| Feb. 23 |
No TeleSeminar --
10th
Annual ERC Site Review Meeting (February 23-24th, Tucson, AZ) |
| March 9 |
Host: Kim Ogden, Chemical
and Environmental Engineering, University of Arizona
Presentation by: Dr. James Beckman, Associate Chair, Chemical
Engineering, Arizona State University
Topic: "CMP Water Reclamation by 'DewVaporization' "
Abstract: The dewvaporation technique will be described with
theory and examples of operations concerning desalination and CMP water
reclamation. (PDF) |
| March 23 |
Host: Ara Philipossian, Chemical and Environmental
Engineering, University of Arizona
Presentation by: Darren DeNardis, Chemical and Environmental
Engineering, University of Arizona
Topic:
"Characterizing Copper - Hydrogen Peroxide Film Growth and Dissolution
Kinetics for Application in Multi - Step Chemical Mechanical Planarization
Models"
Abstract: As copper chemical mechanical planarization
(CMP) modeling efforts become increasingly more sophisticated, it is
important to understand the individual steps that have been found to play
integral roles in the removal process such as oxidation, film dissolution,
abrasion, and dissolution of byproducts. This study focuses on copper
oxidation using H2O2 and copper oxide dissolution,
the two chemical steps of the proposed 3 – step CMP removal rate model.
Ellipsometry, atomic adsorption, scanning electron microscopy (SEM), and
X-ray photoelectron spectroscopy (XPS) techniques were used to
characterize copper film growth characteristics. Ellipsometric results
complemented microbalance results concluding that copper film growth
occurs in hydrogen peroxide solutions of varying concentrations at pH 5.
The film growth profile for 1 weight percent hydrogen peroxide reaches a
saturation point at approximately 500 Å after about 12 hours. SEM images
reveal that 50 – 100 nm copper oxide/hydroxide crystals are observed for t
< 60 minutes and 200 – 300 nm crystals are formed at 24 hours, indicating
that reactions that occur at the fluid – film interface may contribute to
film growth. XPS spectra indicate Cu(I) and Cu(II) oxides and Cu(OH)2
for t < 5 minutes and only cupric oxide and hydroxide for t > 10 minutes.
Copper oxidation studies were performed as a function of temperature in
the range encountered in CMP to determine parameters, which allow
oxidation rates to be calculated a priori for use in the removal
rate model. Copper oxide dissolution experiments were also performed at
multiple temperatures to characterize the dissolution step in the model.
Rates of copper oxidation were found to be higher than dissolution rates
which verifies the cyclic passivation – removal mechanism generally
believed to govern metal CMP processes. (PDF) |
| April 6 |
Host: Christopher Ober,
Cornell University
Presentation by: Yi Yi, Post Doctoral Associate, Ober
Research Group, Cornell University
Topic: "Development
of Novel non-PFS Based Potoacid Generators and Their Performance"
Abstract: The technology of chemically amplified photoresists has
greatly facilitated the development of semiconductor industry. Even in 193
nm (dry and immersion) and next generation lithography (NGL), it is a viable
approach to fabricate the continuingly reduced feature sizes demanded by the
ITRS Roadmap. Among the chemically amplified resist (CAR) systems, the
photoacid generator (PAG) is a critical component. Its function is to
generate strong acid to catalyze the chain reactions in the exposed area of
the resist film.
Although perfluorooctyl sulfonic acid (PFOS) is widely used by the
semiconductor industry in surfactants, top ARCs and PAGs, it is highly
persistent in the environment and has a strong tendency to bioaccumulate.
PFOS containing chemicals are today distributed world wide, even in arctic
areas. They are also found in humans and animals. Based on environmental,
health and safety (EHS) concerns, the EPA proposed a significant new use
rule (SNUR) for PFOS during the year 2000. Moreover, as PAGs based on PFOS
have some disadvantages such as segregation in a polymer matrix, and high
absorption at extreme ultraviolet wavelengths, it is necessary to find PFOS-free
compounds as candidates for PAGs.
In this talk, Cornell’s efforts to develop a series of novel PAGs having
significantly lower fluorine content to address the EHS issues will be
presented. The performance of the novel functionalized PAGs using E-beam and
EUV lithography will be discussed. (PDF) |
| April 20 |
Host: ****** CANCELLED |
| May 4 |
Host: Duane Boning, MIT
Presentation by: Sarah Jane White, Environmental
Engineering, MIT
Topic:
"Developing
Predictive Tools for the Early Environmental Assessment of New Semiconductor
Material"
Abstract: There have been many
instances in recent history where the development of a new compound has done
wonders for its intended purpose, but has also caused unintended, and
ultimately costly, effects on the environment. Prominent examples are the
insecticide, dichloro-diphenyl-trichloroethane (DDT), and the class of
etchants, perfluorocarbons (PFCs). Compounds like these not only damage
the environment, but may in the long run result in lost time, money,
and even liability, for the industries that produce them.
In order to avoid problems like this in the future, researchers must include
environmental considerations in the EARLY stages of their work, before a
product or process has been put into wide use. The real problem then
becomes: what is the best way to assess the environmental impact of novel
technologies?
The goal of this talk is to address the environmental assessment of new
materials in the industry. I will discuss one approach, the comparison of
natural fluxes with anthropogenic fluxes at both local and global scales, in
order to make a first approximation about how a material will affect the
environment. I will also discuss the specific example of lead, how it can
give us some insight into whether this approach will work, and what other
predictive considerations it illuminates. (PDF) |
| May 18 |
Host: Yoshio Nishi,
Director, Stanford
Nanofabrication Facility, Department of Electrical Engineering, Stanford University
Presentation by: Jungyup Kim,
Materials Science and Engineering,
Stanford University
Topic: "Study
of Germanium Surface for Wet Cleaning Applications"
Abstract:
Germanium is an important substrate with high mobility device applications.
Therefore effective Ge surface preparation needs to be developed for
successful implementation of this high mobility substrate. Fundamental
properties of the Ge surface including the etch rate and surface roughness
have been investigated. A new method for surface roughness improvement has
been developed for Ge sirfaces. Surface passivation has also been has been
developed using aqueous hydrogen halides to prevent atmospheric oxidation of
the cleaned surface. Strategy for efficient Ge surface cleaning will be
discussed in comparison to Si surface cleaning. (PDF) |
| June 1 |
SUMMER BREAK |
| June 15 |
Host: Anthony Muscat,
University of Arizona
Presentation by: David
Smith, Vice President, Technology Futures, Inc.
Topic: “The
Future as Seen through Technology Laws”
Abstract: Semiconductor
manufacturing and research are going through dramatic changes in both form
and substance. Global forces, technology, values models, and process models
are all reshaping technology at an unprecedented rate. This talk presents
Technology Futures' forecasts and relates our experiences working with
Fortune 100 companies, government agencies, and emerging companies. Using a
futuristic view grounded in action, this gives the audience the elements
necessary to be a leader in the new manufacturing paradigm. (PDF) |
| June 29 |
Host: Steve Beaudoin, Purdue
University
Presentation by: Steve Beaudoin, Purdue University
Topic:
"Particle Adhesion to Photomasks"
Abstract: Particle adhesion to advanced photomasks is of
considerable interest to the microelectronics industry, as the materials
used in advanced masks are often not compatible with existing cleaning
methodologies. At the same time, the feature scales of future devices are
such that the minimum size above which a contaminant particle on a mask will
produce unacceptable defects is decreasing. The net result is that the need
to understand particle adhesion to masks is very great. In this discussion,
the adhesion between model particles, including alumina, polystyrene and
silicon nitride (primarily) against a number of advanced mask materials will
be presented. Particle sizes ranging from microns to 10s of nm will be
evaluated in dry air, in DI water, and in aqueous ammonium hydroxide.
Experimental and theoretical descriptions of the adhesion will be presented.
[Gautam Kumar, Ravi Jaiswal, Shanna Smith and Steve Beaudoin, Purdue
University, School of Chemical Engineering] (PDF) |
| July 13 |
Host: Alan C. West, Professor,
Department of Chemical Engineering, Columbia
University
Presentation by: Alan West, Columbia University
Topic: "Practical
and Theoretical Considerations for Cu-eCMP"
Abstract: Numerical simulations are employed to demonstrate the
daunting challenges involved in utilizing electrochemical polishing
technologies as an alternative to low-down-force chemical mechanical
planarization. Results show that small-aspect ratio topographical features
sitting on a relatively thin overburden are impossible to planarize by
conventional electropolishing. Electrochemical-mechanical planarization (E-CMP),
where an anodic dissolution process is coupled with a polishing pad, is a
more viable approach. Numerical simulations are compared to results
obtained by E-CMP, as a means of establishing an effective diffusion-layer
thickness and as further demonstration of the infeasibility of
electropolishing. Finally, preliminary designs for a benchtop E-CMP tool
are presented. (PDF) |
| July 27 |
Host: Yun Zhuang and Ara Philipossian,
University of Arizona
Presentation by: Robert Meagley, Sr. Staff Scientist,
Intel Corporation's Researcher-in-Residence,
Lawrence Berkeley National
Laboratory
Topic: "Beyond PFOS- Preorganized Lithographic
Materials at Intel's Molecules for Advanced Patterning Program"
Abstract: The
Molecules for Advanced Patterning Program was initiated at the Lawrence
Berkeley National Laboratory Molecular Foundry by Intel in January of 2005.
Here, we have created several prototype lithographic materials that use
preorganization and self assembly to control photochemical catalysis. This
has profound implications for materials that can restrict pattern blur
without employing the PFOS molecule. It is anticipated that materials that
anticipate patterning geometry and dimensionally decouple patterning
information are possible. (PPT) |
| Aug. 10 |
Host: Reyes Sierra,
Chemical & Environmental Engineering, University of Arizona
Presentation by: Victor Gamez, Chemical & Environmental
Engineering, University of Arizona
Topic: “Biological Removal and
Recovery of Copper in CMP Effluents”
Abstract:
Copper chemical
mechanical planarization (CMP) is rapidly expanding and replacing other
traditional processes generating great quantities of wastewater requiring
treatment. Physico-chemical methods for the removal of copper in CMP
effluents are often expensive and energy intensive. Environmental
biotechnologies offer an interesting potential for the treatment of such
wastewater. Biological treatment provides the possibility to remove organic
contaminants along with copper; meeting regulatory challenges associated
with copper in CMP effluents. The goal of this research is to investigate
the feasibility of an
innovative system configuration that combines a crystallization reactor and
a sulfate-reducing anaerobic bioreactor for the simultaneous removal of
copper and organic matter in a simulated semiconductor effluent wastewater.
Removal of
copper is achieved by precipitation with biogenic sulfides produced by
sulfate reducing bacteria inside an
expanded granular sludge bed (EGSB) bioreactor.
Heavy metals are then deposited in sand granules inside a separate
fluidized bed containing fine
sand (crystallization reactor). The sand offers surfaces for the nucleation
of metal sulfides and subsequent crystal growth. Metal sulfides can then be
recovered from the sand granules in a purified form. (PDF) |
| Aug. 24 |
Host: Christopher Ober,
Vice President IUPAC Polymer Division, Francis Bard Professor of Materials
Engineering, Materials Science & Engineering, Cornell University
Presentation by: Nelson Felix, Materials Science &
Engineering, Cornell University
Topic: "Achieving Small Dimensions with an Environmentally
Friendly Solvent: Photoresist Development Using Supercritical CO2"
Abstract: For more than a decade the idea of using
supercritical fluids in semiconductor processing has been actively explored
by many researchers. With its low critical temperature, zero surface
tension, and non-polar inert character, supercritical CO2 (scCO2)
shows great potential for its ability to process sensitive materials with
patterned features on a very small length scale. However, except for
fluorinated or small non-polar compounds, most materials traditionally used
by industry show poor solubility in this solvent. We will discuss prior
attempts to modify polymeric photoresists to impart scCO2 solubility as well
as explore the range of solvent additives that have been used to alter the
solvating power of scCO2. We will then demonstrate some recent successes in
developing photoresist features in the sub-65 nm range with only scCO2 as
the developer solvent. (PDF) |
| Sept. 7 |
Host: Jim Farrell,
Department of Chemical and Environmental Engineering, University of
Arizona
Presentation by: Atashi Mukhopadhyay, Department of
Chemical Engineering, Stanford University
Topic: "Quantum Chemical Simulation of Atomic Layer
Deposition of HfO2"
Abstract: Atomic layer deposition (ALD) has recently
gained interest because of its suitability for fabrication of conformal
films with thicknesses in the nanometer range. A comparative investigation
of surface hydroxylation states of different surface of m-HfO2 is
central to a better understanding of how precursors react on these surfaces
under ALD growth conditions. We used a combined approach of density
functional theory (DFT) and thermodynamics to determine structures of
anhydrous and hydrated surfaces. Our calculations predict that the
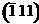 face of the monoclinic phase has the lowest surface
energy and most stable face. The total density and partial density of states
of the monoclinic surfaces exhibit a surface state corresponding to the
surface O-2s states in the inner valence band region. We find that the water
adsorption energy decreases with increasing surface coverage, however the
energetics of the hydration process can be significantly different on
different surfaces. Our investigation showed that under ALD working
conditions the (001) surface retains a higher concentration of Brønsted
acid sites compared to the thermodynamically stable face of the monoclinic phase has the lowest surface
energy and most stable face. The total density and partial density of states
of the monoclinic surfaces exhibit a surface state corresponding to the
surface O-2s states in the inner valence band region. We find that the water
adsorption energy decreases with increasing surface coverage, however the
energetics of the hydration process can be significantly different on
different surfaces. Our investigation showed that under ALD working
conditions the (001) surface retains a higher concentration of Brønsted
acid sites compared to the thermodynamically stable
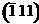 surface due to the higher adsorption energy of water on
the (001) surface. (PDF) surface due to the higher adsorption energy of water on
the (001) surface. (PDF) |
| Sept. 21 |
Host: David Mathine,
University of Arizona
Presentation by: David Mathine, University of Arizona
Topic: "Cell-Based
Biosensors for Toxicity Testing of New Chemicals"
Abstract:
The rapid
development of new chemicals makes the current approach to toxicity testing
unrealistic since the testing relies on laborious and expensive animal
testing. One approach to increase the throughput of toxicity testing is to
use the physiological responses from cells after exposure to an unknown
chemical. Cells from the heart, kidney, prostate, liver, and other organs
can be used to test the responses to these various cell types.
We will discuss the use of a CMOS chip to provide a variety of sensors that
can monitor the cells health in real time. Direct attachment of the cells
to the sensors surface is critical for the development of a reliable sensor
and data showing cell attachment to a variety of semiconductor and insulator
surfaces will be shown. Also, we will discuss our approach to temperature
control of the fluidic environment, which is important to maintain the cell
cultures. (PDF) |
| Oct. 5 |
Host: Farhang Shadman, University
of Arizona
Presentation by: Carl Geisert, Principal Engineer,
Intel Corporation
Topic: " High Purity Process Gas Related Challenges in
Semiconductor Manufacturing”
Abstract: Many publications and roadmaps have recommended a
ppt level of purity in process gases at the 90, 65 and 45nm technology
nodes, along with the in-house analytical capability to measure it. Process
gas related issues experienced during the recent startup and ramp of a
300mm, 65nm high volume fab in Chandler Arizona would suggest that ppb or
even ppm levels of contaminants are a more realistic requirement and what we
typically experience in manufacturing.
Process Gas supply chain reliability, supplier analytical capability, and
internal factory matching requirements have proven to be a much larger
challenge to manufacturing. We will discuss the typical fab distribution
system for bulk gases (O2, N2, H2,..), common gas contaminants and their
sources, and our ability (inability) to measure these contaminants prior to
delivery and in the distribution system. (PDF) |
| Oct. 19 |
Host: Duane Boning, MIT
Presentation by: Dr. Dr.
Rao Yalamanchili, Cleans Product Group, Applied Materials
Topic: "Wafer Cleaning
in Semiconductor Manufacturing: The Single-Wafer Inflection Point"
Abstract: One of the oldest but most prevalent
technologies in semiconductor manufacturing is wafer cleaning, which is used
to remove contaminants from wafers without damage or corrosion and with
minimal material loss. Cleaning processes are tailored to the preceding or
subsequent manufacturing steps, using chemical and physical removal methods
followed by a dry cycle. Technology nodes of 45nm and below are requiring
fundamental changes to wafer cleaning technologies. A main trend is the
switch from the historical bench tools that process batches of wafers
simultaneously to single-wafer systems. New challenges are driven by the
need to have cleaning processes that are effective over one or two minutes,
versus 10-20 minutes. This has resulted in a growth of new chemistries,
combinations of chemistries, and new hardware innovations in the
semiconductor equipment industry. These challenges are being met by an ever
increasing pool of process engineers that must have interdisciplinary
backgrounds in chemical engineering, surface/interface engineering, and
chemistry. In addition, integration engineers must understand the effects of
cleaning in much greater depth because of its impact on key process modules
such as high-K gate oxide implementation, strain engineering, and low
dielectric constant integration for copper interconnects. (PDF) |
| Nov. 2 |
Host: Reyes Sierra, University of
Arizona
Presentation by: Valeria Ochoa, Chemical and Environmental
Engineering, University of Arizona
Topic: "Processes for the
Removal of Perflurooctane Sulfonate (PFOS) from Semiconductor Effluents"
Abstract: PFOS and other perfluoroalkyl sulfonate
surfactants (PFAS) are critical components in a variety of photolithography
and semiconductor manufacture processes. Perfluorinated surfactants are
under investigation as emerging pollutants due to recent reports of their
world-wide distribution, environmental persistence and bioaccumulation
potential. Literature data on the removal of perfluoroalkyl sulfonates is
very limited. Treatment techniques to eliminate these ubiquitous
contaminants from industrial effluents are needed to minimize environmental
discharges. This study evaluated the effectiveness of three approaches for
the removal of PFOS from semiconductor effluents: reductive dehalogenation,
activated carbon adsorption and biosorption. (PDF) |
| Nov. 16 |
Host: James
Watkins, Director of NSF Center for Hierarchical Manufacturing, Co-Director
of MassNanoTech, Polymer Science and Engineering Department, University of
Massachusetts
Presentation by: Ken Carter,
Associate
Professor, Polymer Science & Engineering,
University of Massachusetts
Topic:
“Imprint
Lithographic Techniques for Micro and Nano Patterning”
Abstract: Contact lithography techniques, such as
imprint lithography show great promise in the ability to transfer nanoscale
patterns in a efficient, economic fashion. We have found the networks
composed of a mixture of photopolymerizable monomers (acrylates and
methacrylates) can be molded and photocured, providing image transfer. We
have been exploring the modification of the photopolymer network
composition, incorporating functional co-monomers (imimer, etc.) that allow
for secondary modification of the patterned surface. We have demonstrated
graft polymerizations from patterned surfaces and the ability to adjust
feature sized and chemical functionality in the nanometer size regime.
Additionally, we have been exploring metal/polymer interfaces of the
patterned networks and subsequent modification of network functionality.
These patterned materials are finding utility as high contrast resists and
templates for electronic test structures. The synthesis, characterization
and use of these new materials are discussed. The materials budget of
imprint lithography compared to conventional photolithography can introduce
environmental benefits as there are fewer chemical intensive process steps,
such as rinsing and developing of exposed resists. (PDF) |
| Nov. 30 |
Host: Karen Gleason,
Alexander and
I. Michael Kasser Professor of Chemical Engineering and Associate Director
of the Institute for Soldier Nanotechnologies (ISN), MIT
Presentation by: Karen Gleason, MIT
Topic: "Density functional
theory applied to the Chemical Vapor Deposition of Low Dielectric Constant
Materials"
Abstract: One of the main challenges in designing a low
dielectric constant material to replace silicon dioxide with the class of
materials referred to as organosilicate glasses (OSGs) has been to decrease
the dielectric constant with the addition of alkyl functionality while
maintaining the structural strength of the Si–O network. Molecular
simulation of possible OSG precursors is important to allow for intelligent
precursor selection for robust OSG thin film deposition via CVD. Density
functional theory is a useful tool in understanding and subsequently
controlling the initial chemistry in the CVD process and also for predicting
the 29Si NMR chemical shifts of a variety of organosiloxane
moieties including monomers or precurors for polymerization and
representative segments of organosiloxane polymers or thin films. The
enthalpies of formation and enthalpies of reaction at 298 K for a set of
Si:C:O:H species derived from methylsilanes and methoxymethylsilanes were
computed using the B3LYP density functional theory. Bond strengths and
reactions with O atom and H atom are examined in the context of
understanding the initial reactions in chemical vapor deposition. The Si–H
bond was calculated to be 8.4 kcal/mol stronger than the Si–C bond in
methylsilanes and to increase by 0.6 kcal/mol with increased methylation;
however, the thermochemistry of methylsilane reactions with O atom favors
scission of the Si–H bond to produce hydroxyl and methylsilyl radicals.
Thermodynamic control over the reaction pathways of methoxymethylsilanes is
possible only when considering the reaction with H atom for which
methoxymethylsilanol formation is favored. This illuminates a conceivable
strategy to control the Si–O–Si bonding network while retaining methyl
functionality in a CVD thin film by controlling the ratio of methoxy
functionality and free hydrogen in the reactor. (Thomas
B. Casserly and Karen K. Gleason) (PDF) |
| Dec. 14 |
Host: Farhang Shadman, Chemical &
Environmental Engineering, University
of Arizona
Presentation by: Junpin Yao and Harpreet Juneja, Chemical & Environmental
Engineering, University of Arizona
Topic: "Interactions of Moisture with Dielectric Films"
Abstract: The interactions of
moisture with low-k (blanket and etched/ashed p-MSQ) and high-k (HfO2,
ZrO2) films were investigated by applying a unique experimental
set up with mass spectrometers for real time characterization of moisture
uptake and removal. Process models were developed that provides information
on the dynamics of moisture adsorption and desorption in these films. The
effect of etching, ashing and cap layer on moisture uptake and removal in
these films was also investigated. Usefulness of these models was
illustrated by developing an optimum purge recipe that would help in
minimizing gas and energy consumption and the required purge time. (PDF) |
| Dec. 28 |
No TeleSeminar -
CHRISTMAS HOLIDAY |
|
